Density vs Specific Gravity vs Degrees Plato
Between density, specific gravity, and degrees Plato, brewers have many different ways to measure the sugar content of their worts… maybe too many? Let’s pit these density scales head to head to see what they’re really made of!
Density vs. Specific Gravity
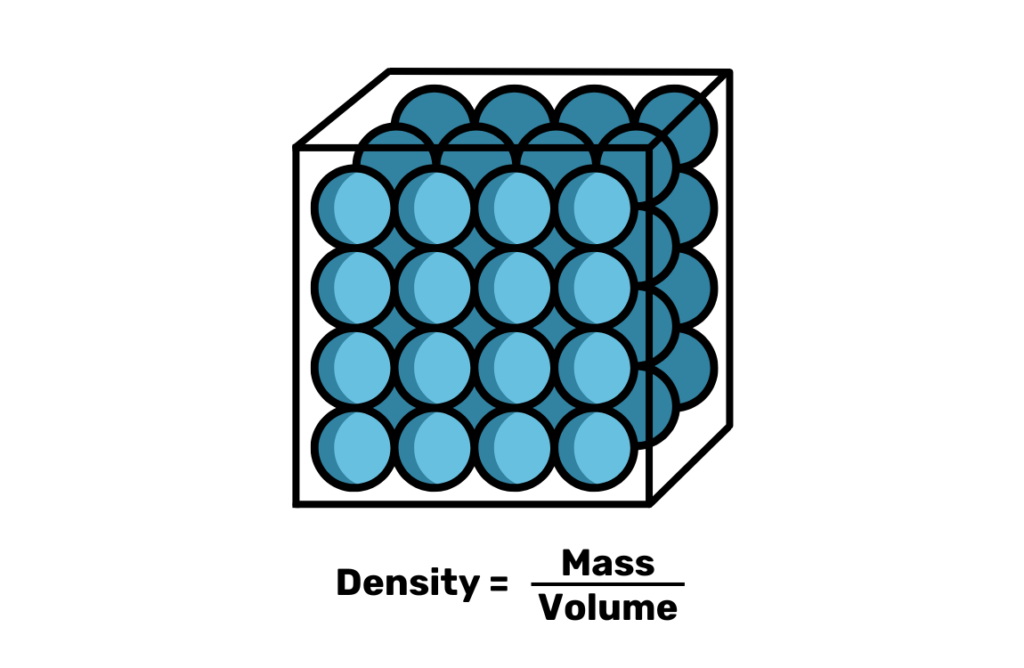
Up first, we have density and specific gravity (SG). Density, like you probably learned in chemistry class, is simply defined as mass over volume for a given substance. It has units like g/cm^3 or g/mL, and is the starting point for some of the other measurement systems we’ll talk about. Although it’s the most explicit measurement strategy, a density measurement alone is hard to use in brewing equations and is difficult to measure without advanced equipment. Therefore, it’s more convenient to use other scales like SG and Plato, which only require basic equipment and techniques to measure liquids against pure water and include methods to correct for temperature.
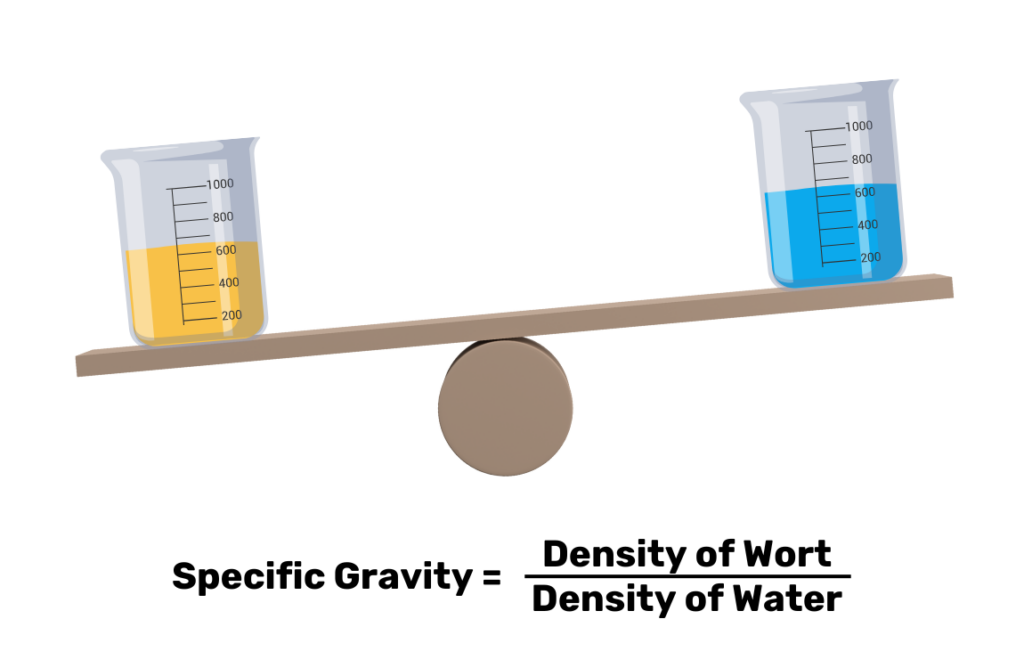
Specific gravity, also called relative density, isn’t a discrete measurement of a substance on its own. Instead, it’s a dimensionless or unitless measure of the substance’s density compared to another substance. For brewers, the reference liquid is water, and the specific gravity value obtained is simply a ratio of the wort density to pure water. For example, if your wort specific gravity is 1.045, that means it’s 1.045 times denser than water. By measuring against pure water, brewers can quickly determine how much extract is in their worts and therefore how much alcohol will be produced.
However, there’s one very important thing to keep in mind when measuring densities and gravities: temperature. As the temperature of a fluid changes, its density will change as well. This is especially true for brewers, where wort temperature can be anywhere between 68°F (ready for fermentation) or 212°F (during the boil). To get around the temperature problem, measuring devices are calibrated for a specific temperature at which they’ll provide an accurate reading. For most hydrometers, this is room temperature, aka 20°C.
Hydrometers
A hydrometer is a graduated glass tube that floats at a height relative to the liquid’s density. To read it, simply read the gradation at the meniscus of the liquid level. Then, get the temperature of the liquid and correct the gravity value. Most hydrometers will have a correction scale printed on it, but there are also temperature correction calculators online available for this.
So why bother with specific gravity if it is the same thing as density in the brewing context? Why not just use density? Because of the equipment used to measure each. More advanced instruments can measure the density of a liquid directly. These instruments are more accurate than a hydrometer or refractometer, but are also much more expensive, which puts them out of reach for smaller breweries. By using a hydrometer to measure density on the specific gravity scale (aka taking into account the aqueous base solution), brewers get a cost-effective measurement that provides enough accuracy to inform brewing calculations and recipes.
Specific Gravity vs. Degrees Plato
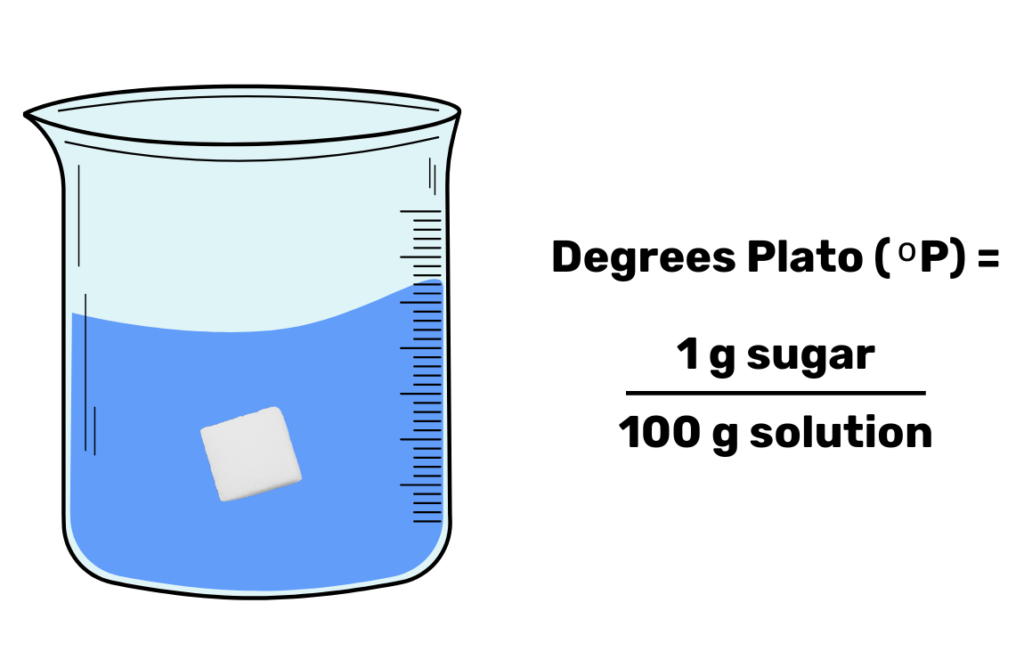
Now that we know why SG is more useful than density when brewing, let’s bring out our next competitor: Degrees Plato (°P). Instead of a relative measurement, Degrees Plato is defined as a weight percent: 1 degree plato is equivalent to 1 gram of sugar dissolved in 100 grams of water. This scale is frequently used on a measurement device called a refractometer, though you can also measure Degrees Plato with a hydrometer.
These scales can be used interchangeably for the most part, especially since modern brewing software will convert between them on the fly. However, there’s one major advantage to using the Plato scale over SG: the Plate scale represents an actual concentration, which makes math easier. Let’s say you have 100 liters of wort at 14.5°P but need to dilute that down to your target starting gravity of 13.0°P. By using a simple dilution equation (c1v1=c2v2) we can determine that the final volume of our wort should be 111.5 liters, or an addition of 11.5 liters of water to reach our target of 13.0°P.
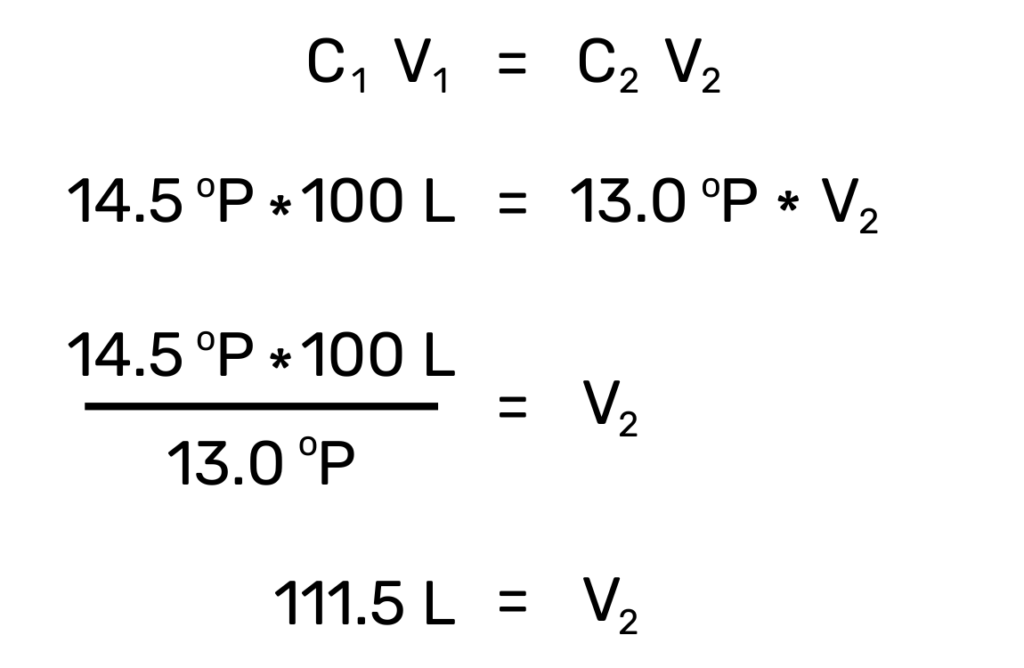

This equation does not work when using the SG scale, since those values are relative densities, not concentrations. Here’s example: let’s say we had 100 liters of very dense wort of 1.080 that we wanted to dilute in half, that is, to 1.040. Intuitively, we know that we simply need to add another 100 liters of water, but using our dilution equation with these SG values does not give us the result we would expect: we get a final volume of 103.8 liters, or an addition of only 3.8 liters! Again, most brewing software programs will handle dilution calculations automatically, but it’s important to know the limits of the density scale you choose.
Refractometers
A refractometer uses the principle of refraction to measure the concentration of sugar: a solution with more sugar will more aggressively bend light and can be correlated to a density scale. However, as with a hydrometer, it’s important that the liquid is at room temperature to get an accurate reading.
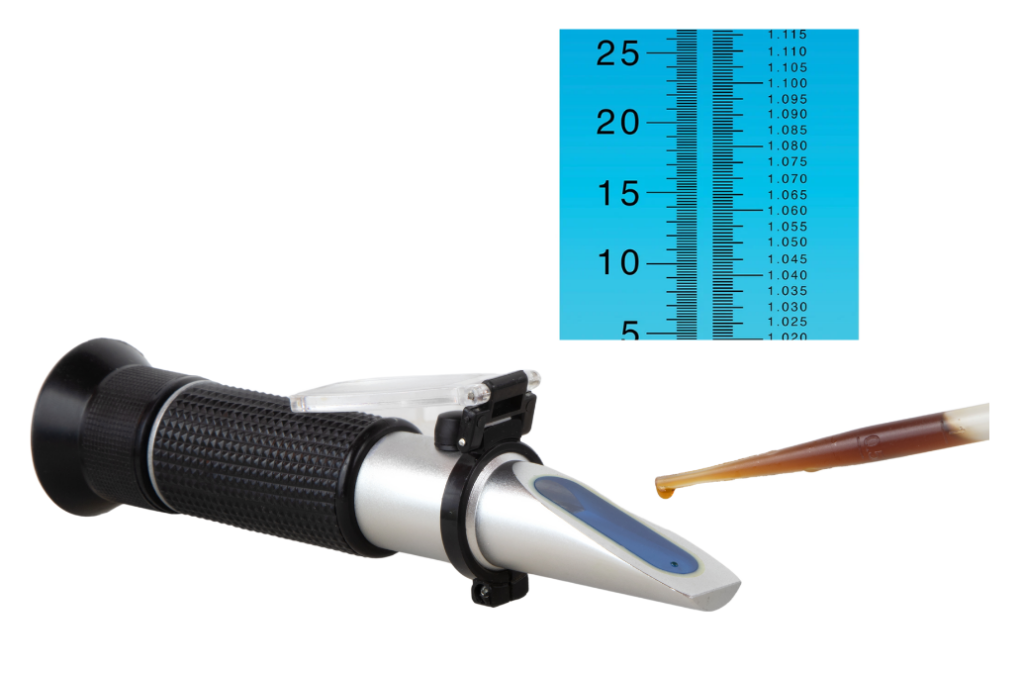
No matter what scale you’re using, by keeping these details in mind you can be sure you’re getting the most accurate data on your brew. Stay tuned for more duels, battles, and showdowns from the Catalyst Club!
