The Not-So-Stinky Perks of Sulfur

Sulfur, let’s chat about all it’s different forms and how they relate to winemaking.
Even with all the various philosophies on SO2 usage and management, it is important to understand the lingo and the chemistry about this commonly used preservative.
Sulfur Characteristics
- Bright yellow, soft, crystalline solid
- Odorless and tasteless in its pure form – though many compounds with sulfur stink! Think skunk spray, matches, and rotten eggs.
- Burns with a blue flame (see video below!)
- Insoluble in water
- Can be found naturally (mined) but the majority is created as a byproduct of natural gas production
- Sulfur is the 5th most common element on earth and makes up 3% of the earth’s mass (that is the size of 2 moons!)
Where Sulfur is Used

- Matches
- Fertilizer
- Anti-fungal spray for agriculture (like vineyards)
- Car batteries
- Tap and bottled water
- Detergents
- Antibiotics (like penicillin)
Sulfides vs Sulfites vs Sulfates
These three terms are often confused with one another, so let’s get the terminology straight.
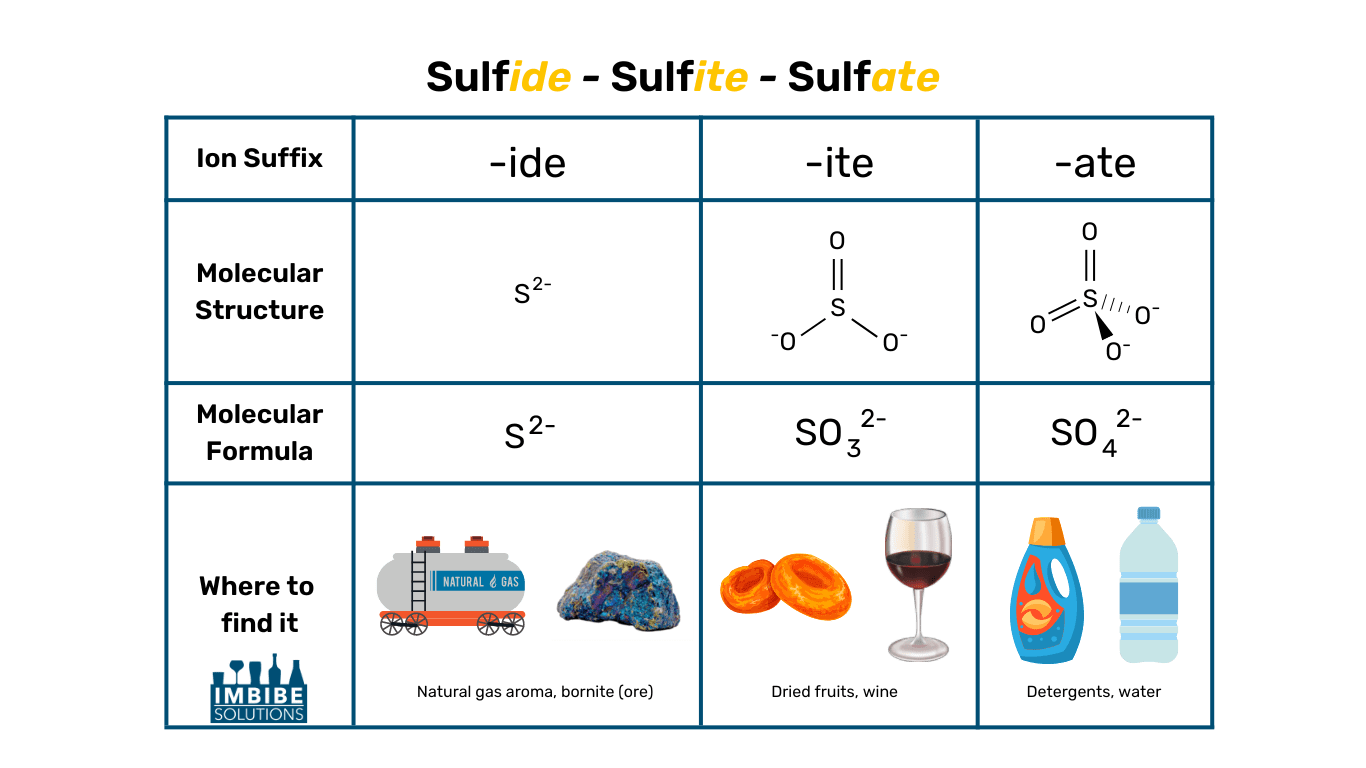
Sulfides (S2-)
- A sulfur anion (extra electrons)
- Forms a salt when bound to a base, for example, Hydrogen Sulfide
- Smells like rotten eggs
- A byproduct of fermentation, highly volatile
- Most major ores of important metals such as copper, lead, and silver are sulfides
Sulfites (SO32-)
- A sulfur oxoanion (anion made of sulfur and oxygen)
- Forms a salt when bound to a base, for example, Potassium Metabisulfite
- Acts as a preservative – keeps food from spoiling and helps maintain color
- Found in: dried fruits, baked goods, canned tuna, wine
- Additive or natural byproduct of fermentation
Sulfates (SO42-)
- A sulfur oxoanion (anion made of sulfur and oxygen)
- Forms a salt when bound to a base, for example, Calcium Sulfate
- Also known as Salts of Sulfuric Acid
- Act as a surfactant between a liquid and another substance (liquid, gas, or solid)
- Used for: Detergents, shampoo, dye, bath salts, water adjustments (brewers’ gypsum)
Now that we’ve got some basic lingo down, let’s talk about sulfur’s application to winemaking.
Using Sulfur in Winemaking
Sulfur is used in winemaking in the form of sulfur dioxide (SO2), a colorless gas. Sulfur dioxide is an antioxidant and broad-spectrum antimicrobial. One probable antimicrobial pathway is the splitting of disulfide bonds in enzymes and regulatory proteins essential to cell metabolism. Binding to nucleic acids, disturbing ATP or NAD production/usage, or changing the intracellular pH may also be factors in its effective antimicrobial capabilities. (Jackson, 437)
Fun Fact
Sulfur was first used by Romans during winemaking to prevent the vinegar smell in empty barrels. We now know that sulfur is an antimicrobial (inhibits bacterial growth). Without large quantities of bacteria present, they were thus preventing the mass production of acetic acid (better known as vinegar).
Sulfur dioxide can be introduced to wine as a gas dissolved in liquid or powdered salt, like KMBS (potassium metabisulfite), which effervesces as it dissolves. You can also burn sulfur wicks in empty barrels to inhibit microbial growth before filling it with fresh wine.
SO2 can be found in solution in either a free or bound form. SO2 may bind with sugars, phenolics, aldehydes, and other compounds found in the wine. Some of these compounds may fall out of solution as solids while others will stay in solution and change the nature of the compound they bound to. For example, some aroma and flavor compounds may alter or even become odorless.
The free form can be found as three species that sit at equilibrium in an aqueous solution: sulfur dioxide (SO2), sulfite ion (SO32-), and bisulfite ion (HSO3–). The concentration of each is driven by the pH, as seen in the chart below.
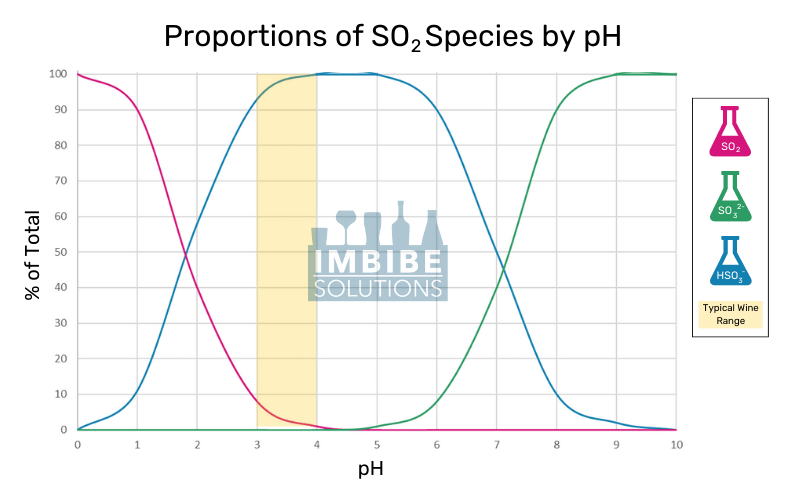
Sulfur Dioxide is the most active form as it is an effective antimicrobial and antioxidant. Bisulfites aid in reducing oxidative browning reactions and scrubbing hydrogen peroxide (H2O2). Sulfites have some reactive capabilities but are much less efficient than their counterparts.
Molecular SO2
Since the concentration of each species is very pH dependent, the use of Molecular SO2 (vs free or bound) comes in handy. Molecular SO2 is a calculated value that portrays the efficacy of the sulfur dioxide in solution accounting for a wine’s pH.
A molecular SO2 between 0.5 – 0.8 ppm is considered sufficient for yeast and bacteria inhibition in wines. Reds can often employ lower levels in this range because they are usually higher in alcohol and phenolics (additional antimicrobial agents) than white or roses.
To calculate the molecular SO2, use the following equation.
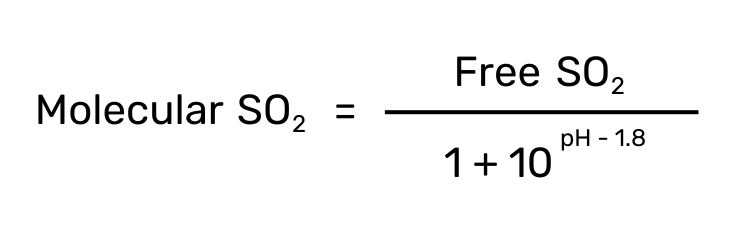
“Contains Sulfites”
So, if sulfur dioxide is the primary preservative, why do wine bottles contain the disclaimer “contains sulfites”?
The Code of Federal Regulations (21 CFR 101.100 (a)(4)) states, “…any sulfiting agent (sulfur dioxide, sodium sulfite, sodium bisulfite, potassium bisulfite, sodium metabisulfite, and potassium metabisulfite) that has been added to any food…” in a detectable amount greater than 10 ppm must be declared. It was found that a small portion of the population has an allergy to the above, commonly-used-in-food sulfites and they must be listed like other allergens (eggs, nuts, soy, etc.).
While sulfur dioxide itself is not a sulfite, we also know that it can be found in equilibrium with sulfites in wine and may contribute to the presence of sulfites in the final product. So in short, same same but different (as far as the US government is concerned).
Total SO2
The last piece of the sulfur puzzle for wine is that of total SO2. Total SO2 is the sum of free SO2 and bound SO2. It is important to measure the total SO2 before packaging to ensure compliance with federal limitations. In the United States and Europe, there is a 350 ppm cap on the amount of SO2 that can be present in wine (free or bound).
While most wines stay far below this limit (closer to the 100 ppm range in Virginia), it is important to be aware of your wine’s concentration. High concentrations can lead to color stripping, aroma and flavor distortion, and sulfur-like odors.
Why measure Free and Total SO2 ?
References
“Sulfur Dioxide.” Wine Science: Principles and Applications, by Ronald S. Jackson, 5th ed., Academic Press, Elsevier, 2020, pp. 434–440.
“Sulfur.” Chemicool Periodic Table. Chemicool.com. 18 Oct. 2012. Web. 11/18/2022 <https://www.chemicool.com/elements/sulfur.html>.
