Water 101

The Four Elements: Water
How to use the power of water chemistry
to level up your next brew!
Water, malt, hops, and yeast. Together, the four elements combine and make humanity’s favorite beverage: beer! Water is perhaps the most fundamental—and yet overlooked—element in the brewing process. Understanding its secrets can take your beer from good to fantastic. After all, water makes up around 95% of beer’s volume, so if there’s an issue with your water, there will be an issue with the beer. Let’s dive into all things water chemistry!
Soft water, like surface waters from rivers, contain a small amount of dissolved salts, whereas hard water, like water from aquifers or deep wells, contain large amounts of salts.
A Quick Brewing Water History
Compared to how long humans have been making beer, our understanding of water chemistry is a new development. Until recently, the success of a brewery was largely dependent on the water source that was available—and how well the brewer was able to work with it. In Burton-on-Trent, England, where water sources were “hard”, brewers were able to make fantastic pale ales whose quality depended on that water. And in Pilsen in the Czech Republic, “soft” water sources fostered the creation of an entirely different style: Pilsner. Although brewers have been adjusting the chemistry of their water sources for quite some time, they still have to work with the water profile of their region. But why did the water in Burton-on-Trent work so well for pale ale, while Pilsen’s water worked so well for light lager? The answer is the difference in “soft” versus “hard” water.

In general, the water profile of a given water source is simply the amounts of various ions or compounds present in the water. Soft water, like surface waters from rivers, contain a small amount of dissolved salts, whereas hard water, like water from aquifers or deep wells, contain large amounts of salts. Water hardness is measured in terms of the calcium ion concentration in mg/L, since calcium is the primary ion that contributes to hardness.
Most craft breweries get their water from a local water utility, but some might use water from wells or surface water. Regardless of the source, water that’s used in the brewery needs to be free from microorganisms, heavy metals, and other contaminants like volatile organic compounds. This can require some serious processing for breweries sourcing their own water, but a brewery that gets water from a water utility usually only needs a carbon filter. While a carbon filter won’t remove water hardness or dissolved minerals, it will remove chlorine, which causes off flavors in the final product. After filtering, brewers can then adjust the water profile using salts. Let’s explore the major components of a brewery’s water profile and see what effects they can have on the brewing process!
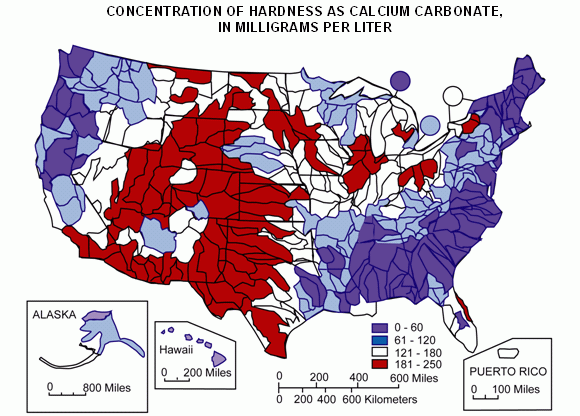
Image from USGS.gov: Mean hardness as calcium carbonate at NASQAN water-monitoring sites during the 1975 water year. Colors represent streamflow from the hydrologic-unit area. Map edited by USEPA, 2005. Modified from Briggs, J.C., and Ficke, J.F., 1977, Quality of Rivers of the United States, 1975 Water Year — Based on the National Stream Quality Accounting Network (NASQAN): U.S. Geological Survey Open-File Report 78-200.
Calcium
Calcium helps to lower the pH and keep it in the right range. The pH level is particularly important in the mash, since the enzymes that convert starch to sugar work best at particular pH values. For most beers, a mash pH of 5.2 to 5.6 is ideal. Calcium can also help to stabilize one of these enzymes, alpha-amylase. In addition, calcium can help with flocculation: the process where proteins or yeast cells clump together and fall out of solution. This is important in both the boil, where the coagulated protein—known as hot break—needs to be removed from solution, and after fermentation, where yeast cells need to be removed so that the beer can be clarified. Calcium ion concentration should be between 20 and 150 ppm.
Fun Fact
Adding salts to brewing water, especially calcium carbonate, is referred to as “burtonizing.” It is an attempt to mimic the naturally hard water in Burton-on-Trent, England.
Magnesium
Magnesium contributes to wort pH like calcium but has less of an effect. It is more important for yeast which need magnesium for some enzyme functions, and brewing water will usually have more than enough for proper yeast health. Magnesium does have a direct effect on beer flavor, and in excess amounts can create a sour or bitter flavor. Magnesium ion concentration should be less than 30 ppm.
Sodium
Sodium can contribute to salty, minerally, or metallic flavors at higher concentrations, but also contributes to the fullness of a beer like many ions do. Sodium ion concentration should be between 75 and 150 ppm.
Common Salts for Adjusting Brewing Water
- Sodium bicarbonate (baking soda)
- Calcium hydroxide (lime)
- Calcium sulfate (gypsum)
- Magnesium sulfate (epsom salt)
- Calcium chloride
- Magnesium chloride
- Sodium chloride
Sulfate
Sulfate is the main anion (negatively charged ion) that is found with calcium and magnesium. It can add a dry, bitter flavor to beer, creating a snappier mouthfeel. However, sulfate levels should be balanced with chloride levels since the ratio between the two ions can have huge effects on beer flavor (more on that later). Sulfate ion concentration can vary widely but should be between 50 and 400 ppm, depending on the style.
Chloride
Chloride ions, in contrast to sulfate ions, contribute a fuller flavor to beer, providing a sweeter or rounder perception. Chloride ion concentration should be between 50 and 150 ppm.
Bicarbonate
Bicarbonate ions (HCO3–) and carbonate ions (CO32–) exist together in solution, and the concentration of each depends on the wort pH. Bicarbonate ions contribute to residual alkalinity and can hold wort pH at high values—not a good thing! Waters high in bicarbonate are therefore good for brewing dark beer, since the heavily roasted malts add acidity that balances the bicarbonate. Bicarbonate ion concentration should usually be less than 50 ppm but can be higher when brewing darker beers.
Fun Fact
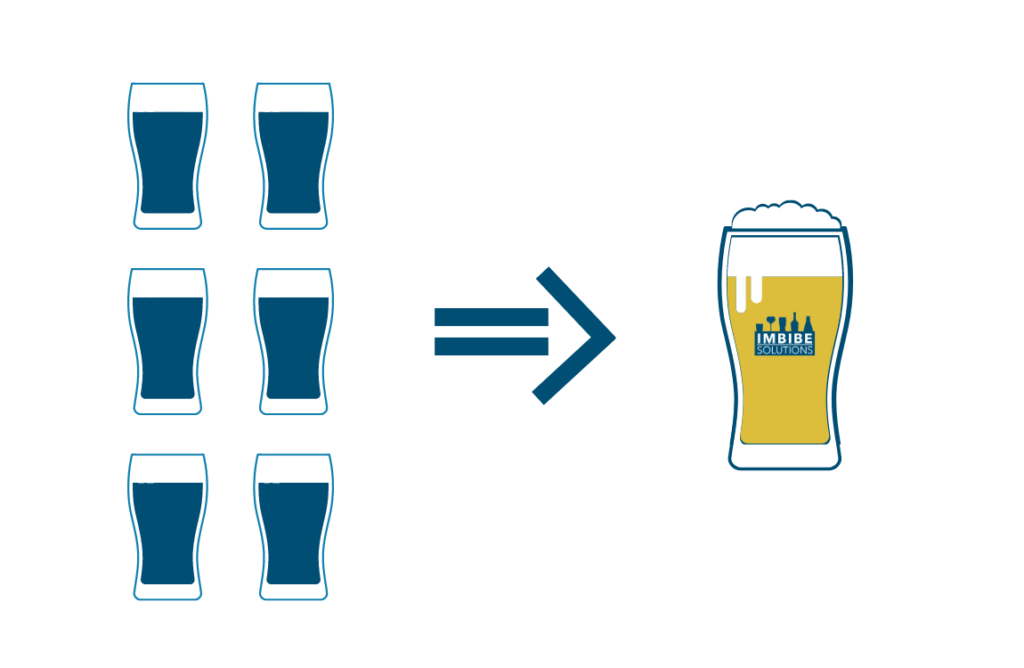
On average, breweries use 6 pints of water to make 1 pint of beer, but some breweries can use as low as 3 pints for each pint produced and some use more than 30!
Sulfate–Chloride Ratio and Total Dissolved Solids
Given the ions listed above, how can brewers adjust their water profile for the perfect pint? The first major parameter is the sulfate–chloride ratio, which is exactly what it sounds like: the ratio of the sulfate ion concentration and the chloride ion concentration. This ratio contributes to the balance between maltiness and hoppiness of the final brew. Sulfate enhances the bitter hop character and chloride enhances the sweet malty character of the beer. The ideal ratio between the two ions depends on the beer style being made: for most beers a ratio between 5 to 1 and 0.5 to 1 sulfate to chloride is ideal, but IPAs can go as high as 10:1. However, adding too much of either can be an issue, and the sum of the two ions should not be more than 500 ppm.
The second parameter is total dissolved solids (TDS), or the total amount of the ions in solution. Beer brewed with unmodified distilled water, or water with low TDS, can taste watered-down and will have no structure. Having the right amount of TDS means your beer will have the right amount of mineral structure and body. There’s no hard and fast rule for TDS for individual beer styles, but in general, lighter beers with more delicate flavors should have less TDS, and heavier beers with a ton of malt or hop character should be supported by a higher TDS.
In order to make informed decisions about your water profile, you’ve got to know what you’re starting with: many water utilities provide a water report, which can provide a good start, but oftentimes these can be misleading and might not reflect the water you’re actually getting. An even better option is to have your water tested for each of the brewing ions which will provide a much more accurate picture of your water.
Once you know what you’re starting with, it’s all about balancing ions and making sure each is in range for the style of beer being made. Online brewing calculators and recipe software can help with these adjustments and can even provide suggested water profiles for each style!

Additional Resources
For a deeper dive on all things water, be sure to check out these resources on water use in the brewery:
- Brewing Science and Practice by Dennis E. Briggs, Chris A. Boulton, Peter A. Brookes, and Roger Stevens, Chapter 3: “Water, effluents and wastes”.
- How to Brew by John J. Palmer, Chapter 21: “Residual Alkalinity, Malt Acidity, and Mash pH” and Chapter 22: “Adjusting Water for Style”.
- For the truly obsessed: Water: A Comprehensive Guide for Brewers by John J. Palmer and Colin Kaminski.
